Pharmaceutical Translation Services
GTS excels in providing pharmaceutical translation services to drug companies that want to grow pharmaceutical sales internationally and extend sales across the member states of the European Union (EU). Our quality policy is compliant with the ISO 17100:2015 standard, which was developed especially for translation services providers. Click here to get an online price quote for pharmaceutical translation services.
Bringing a new drug to market is a lengthy and expensive process. Pharmaceutical companies can spend up to 10 years and more, and over $500 million to get a new drug to market. Since more and more clinical research and drug manufacturing is being done in multiple countries and in multiple languages, quality language services can help bring a drug to market faster and can help streamline the clinical trial process. Translation may be required at many stages, including clinical research, regulatory submission and review, production and marketing.
Submissions to the European Medicines Agency
Before a medicinal product is authorized for sale in the EU, a Summary of Product Characteristics (SmPC) must be submitted to the European Medicines Agency or to the authorities of the member state. The SmPC must be submitted in the language of each member state. Click here to see a list of official languages.
Guidelines for preparing the SmPC documents, in each of the relevant languages, can be found on the EMA website. GTS provides translation of SmPC documents in all of the official EU languages, as well as the languages of the EEA-EFTA states (Icelandic and Norwegian).
Accurate translation of the SmPC document and associated texts is of critical importance. These are the texts that appear on the drug packaging, the labeling (such as vial, bottles, blister and sachet labels) and the Patient Information Leaflet (PIL) which is inserted in the drug package. Translation errors in drug packaging or labeling can result in injury or loss of life.
Navigating the QRD Process
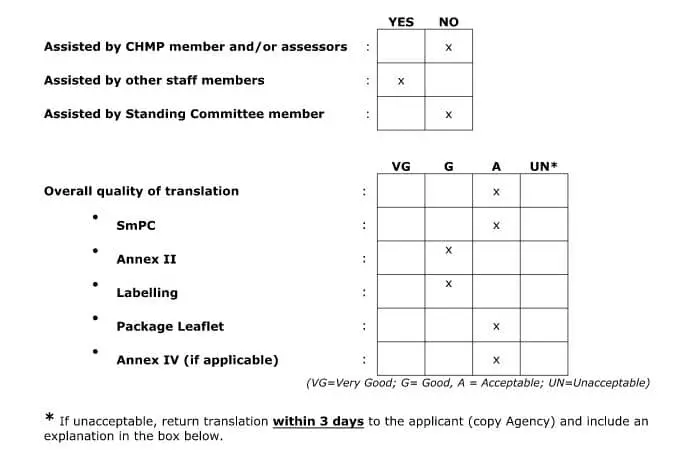
Once the SmPC documents have been submitted to the EMA, the SmPC translations are sent to the Member States (MS) for linguistic review. The review is done by members of the QRD (Quality Review of Documents) team, using templates that can be found on the EMA website. The EMA or its QRD representatives will carefully check the translation to determine if it is acceptable. They will also submit requests for document revision if they deem it necessary. The following is what a typical translation feedback report summary looks like:
Delivering Translations within Agency Timetables
Timely delivery of pharmaceutical translations to the Agency can be tricky, as the time window allowed by the EMA for submitting the translations, after approving the source SmPC document, is very short. Typically, you will only have 5 days to get the translations to the EMA. Make sure to communicate with your translation company early in the process so that they can hit the ground running as soon as you have the Agency’s approval. This will ensure that the Agency gets the translations on time.
Translation accuracy and quality are also paramount, as a poor translation can result in extra delays in the QRD process. GTS has specialized, in-country medical translators in all member states that provide linguistic review and validation. We are familiar with the QRD process and can usually provide translations which are accepted with a minimum of revision requests.
Linguistic Quality Assurance in the DTP Process
After the QRD process has been completed and final approval of the translation have been received, mock-ups of the packaging must be prepared in accordance with Agency guidelines. GTS has proven experience in meeting these requirements and providing effective labeling and packaging. If you are contracting the packing design and artwork from another company, we can provide linguistic QA prior to production.
